CT Severity Score
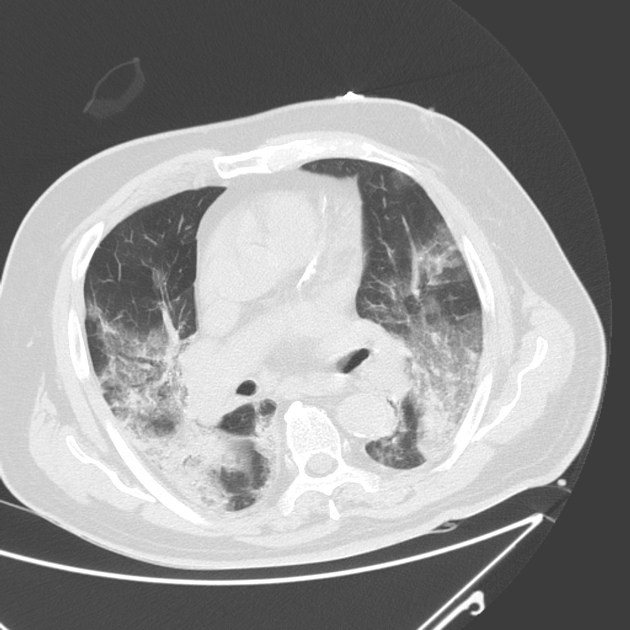
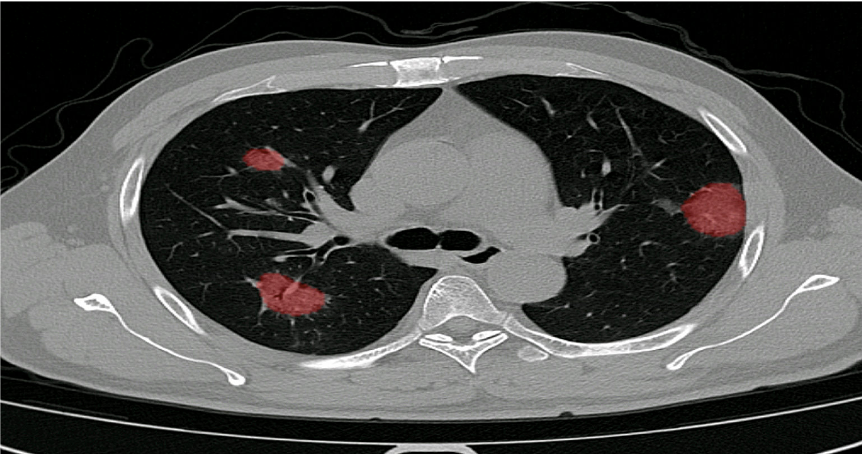
The extent of lung damage caused by COVID-19 seems to be of prognostic value. Thus far, numerous radiological studies on the virus have used the CT severity score as an outcome measure.
The score gives a reflection of the amount of abnormality in each of the five lung lobes. Each lung lobe is given a semi-quantitative involvement score.
Score 1: < 5% involvement
Score 2: 5%-25% involvement
Score 3: 26%-49% involvement
Score 4: 50%-75% involvement
Score 5: > 75% involvement.
How it works
CT scan images of the chest are automatically pseudonymized and sent to the Holland AI platform. On this platform, the images are analyzed and enriched by PulmoFast-CTSS to quantify the CT-Severity Score.
After analysis, the report is automatically added to the PACS-viewer and ready of further analysis.
An API can automatically distribute the score given to other models used in your hospital.
PulmoFast
Distribution & Implementation
PulmoFast-CTSS is exclusively distributed via the Holland AI platform, the global integrated connectivity solution linking cloud-based AI and machine learning applications with legacy hospital infrastructure.
This minimizes operational impact for implementation, whilst maximizing output and enabling deployment of a wide plethora of AI applications to your hospital.
Holland AI Platform for AI distribution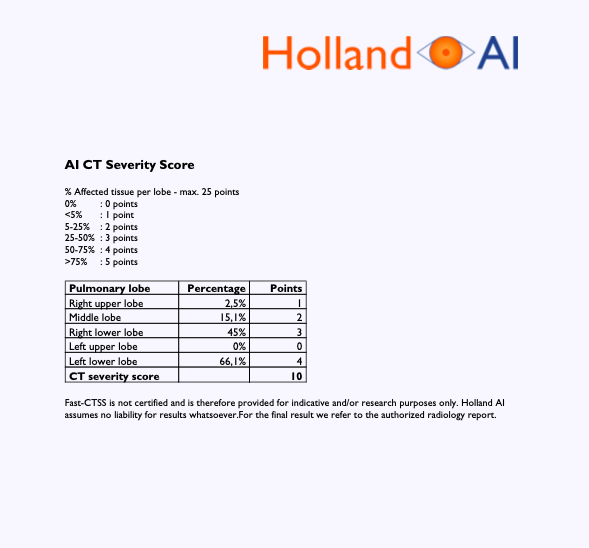
Output
The output will be made visible in any PACS viewer, and the results can be copied into speech dictation software, saving assessment and reporting time for the radiologist.
In addition, the quantitative measures can be used for incorporation into other predictive models using laboratory results and other clinical parameters.
Continuous improvement
PulmoFast-CTSS will be continuously evaluated and improved in collaboration with clinical partners.
Collaboration with clinical partners
Get in touch with us for more information. Fill in the form to apply for PulmoFast-COVID or for further information.
Disclaimer
PulmoFast CTSS is currently undergoing clinical validation and is strictly provided ‘as-is’.
Current use is limited to research purposes only, subject to obtaining an exemption from relevant authorities. Data is processed in line with applicable rules and regulations including GDPR/AVG, and done in a safe and secure manner. Under the current circumstances, the European Commission and various national regulatory bodies have provided an emergency waiver for obtaining regulatory approval (CE), which exemption can be used by Pulmofast0-CTSS, subject to prior notice and approval with the relevant regulatory body.
Get in touch
To support hospitals in their fight against COVID we are offering our application to hospitals. Please reach out to discuss integration, for which we are currently developing a self-installing tool to enable local IT staff to perform integration and installation