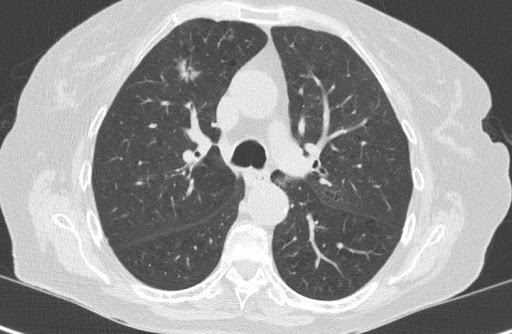
In December 2019, we started clinical validation of PulmoFast, the game-changing tool for computer-assisted detection for pulmonary nodules on CT-scans on the chest. Together with The Maasstad Hospital (Rotterdam, The Netherlands), we are assessing various KPI’s, which have so have been to exceeding expectations.
Furthermore, PulmoFast is currently undergoing regulatory approval in Europe (CE) and the US (FDA).
PulmoFast will be rolled out for further clinical validation in the coming months. After obtaining regulatory approval, PulmoFast will be made available at a competitive transaction-based pricing model, creating greater value whilst simultaneously reducing costs, creating a leap in value for doctors, hospitals and society.