The Maasstad Ziekenhuis (Rotterdam, The Netherlands) will use Artificial Intelligence (AI) to quantify the amount of involved tissue in patients that are possibly infected with the Coronavirus (COVID-19). This is done in close collaboration with Holland AI, a company active in the development and integration of AI-applications in healthcare.
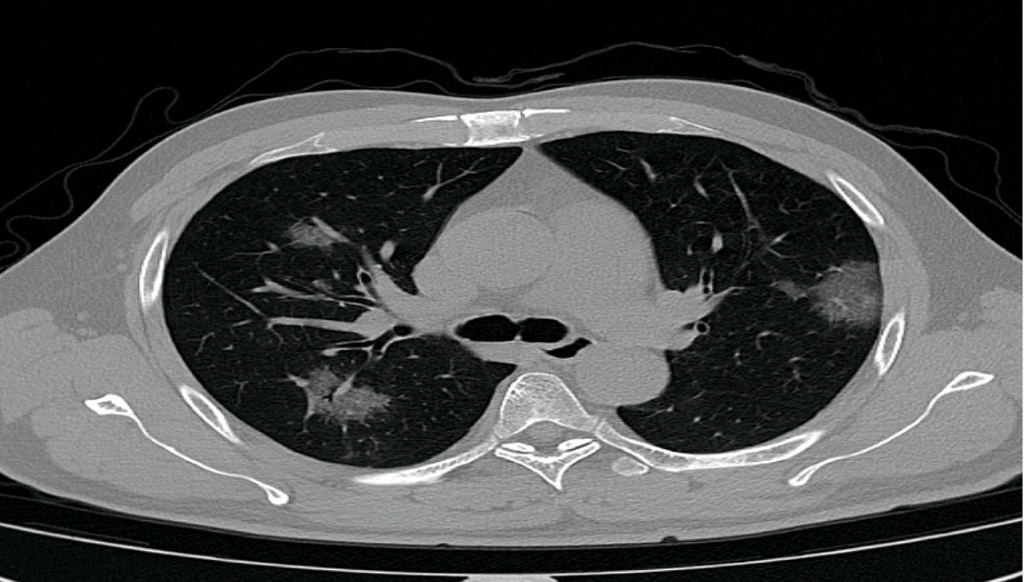
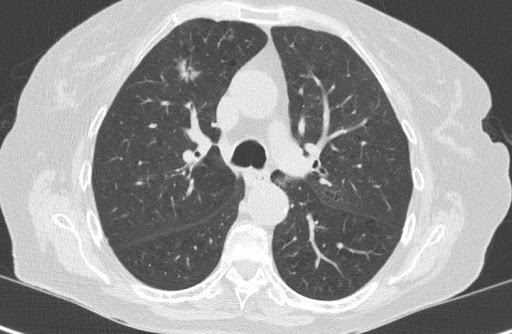
The application, PulmoFast-CTSS, analyzes chest CT-scans and gives an indicative score (CT severity score) of the amount of involved tissue. Previous studies have shown prognostic value of the CT severity score, and thus the score may help clinicians in their treatment choices. The Maasstad Hospital and Holland AI are now working on a clinical study to further evaluate the value of the CT severity score. Based on the outcomes of their analyses, further steps will be taken, for example distribution of knowledge and the application to other hospitals.
“Several companies are joining forces and are freely offering their AI software for research of and the fight against the coronavirus. Just as Holland AI, a start-up company of one of our residents Ayoub Charehbili and his business partner Damiaan Sprenger”, says André Niezen, a radiologist in the Maasstad Ziekenhuis. “The department of radiology and nuclear medicine of the Maasstad Ziekenhuis is proud to – in addition to regular diagnostics – contribute to the diagnosis and treatment of patients contaminated with the coronavirus (COVID-19).”
André Niezen, a radiologist in the Maasstad Ziekenhuis
AI in a supportive role
The radiologist is and stays the person responsible for the analysis of the CT-images, but can be assisted by PulmoFast-CTSS. To make this possible, the AI-software is fully integrated within the existing IT-infrastructure.
Multidisciplinary collaboration
The Maasstad Ziekenhuis will also investigate if it’s possible to integrate the CT-severity score in other predictionmodels (for example with lab test values). For this reason the hospital installed a multidisciplinary crisis workgroup on AI of multiple clinical and diagnostic specialisms, the clinical-chemical laboratory and the department of clinical informatics.
Clearly, the COVID-19 pandemic poses significant challenges for doctors and hospitals. For example, there are major capacity management issues and it possibly very hard choices will have to be made whether to treat a patient or not with only limited prognostic parameters available.
At Holland AI, we have been contemplating various avenues to offer non-profit support. We have added new functionalities to our application for detection of pulmonary nodules, analyzing the extent of lung damage by COVID-19.The main opinion of our team was that we should not engage in developing COVID-19 solutions unless we had the feeling that we could add real value to the scientific work being done on the pandemic. Also, we explicitly don’t want to leverage this crisis for commercial stakes.
Several algorithm vendors have developed (or are developing) algorithms for detection of COVID-19 on chest-CT scans or X-ray images of the chest. These solutions can definitely add value in situations where there is scarcity of radiologists in combination with a high volume of CT-scans.
However, in the Netherlands scarcity of radiologists is – knock-on-wood – not a problem at this moment. In addition, CT-features of COVID-19 are highly aspecific and can also be found in, for example, other types of pneumonias, and it remains unclear if an AI-tool can distinguish pneumonias based on their causative pathogen.
So, where can we add value using AI in this situation? As mentioned before, treatment decisions must be made and there are only a few prognostic parameters available. Several studies have used the so-called CT-severity score to make an estimation of the affected lung tissue in each lung lobe. One can imagine that patients with only 5% affected tissue may have a better prognosis than a patient with 50% damage, presenting on the same day after the onset of complaints. Dutch radiologists are currently reporting this score in the standard COVID-19 reporting template. However, this is an additional task in the ever-growing list of radiologist’s tasks and time-consuming in terms of assessment and reporting.
In addition, the CT-severity output can be used for integration in predictive models using other input data (e.g. blood samples and clinical parameters). In this way, the CT-severity score can important value for doctors (e.g. ICU-doctors and pulmonologists) to decide on the follow-up of treatment.
Considering this, we have decided to tweak and adjust the advanced segmentation capabilities of PulmoFast (our in-house algorithm for detection of pulmonary nodules), and deploy a model to provide quantification of the CT-severity score. The first version of this solution was built in only one week and we are very grateful for the tremendous teamwork. Of course, this is the first version and we are currently fast-tracking a development study in one of our main partner institutions (Maasstad Ziekenhuis Rotterdam). We will offer an API solution for all hospitals in the EU to use this tool free of charge.
How it works:
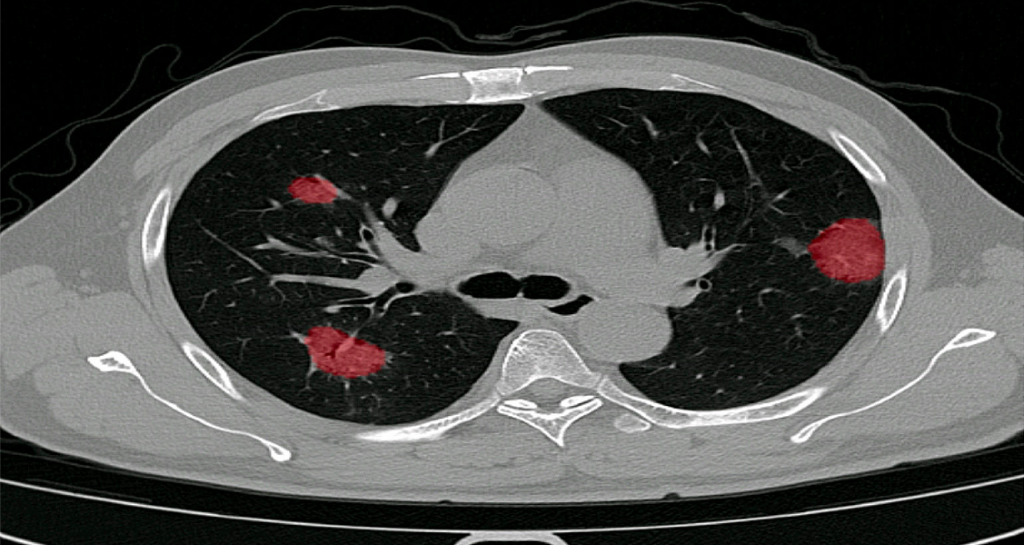
Automated scoring of the extend of damage inflicted on the lung due to COVID-19.
Scoring is shown per individual lobe, consistent with the scores used in numerous clinical studies
Fully integrated into existing workflow and PACS viewer.
Why do we do this from a developer’s point of view? To prove ourselves that we can develop such a solution within 2 weeks, with easy implementation, in full compliance with all relevant law and legislation. And after the crisis, we are happy to talk about how we can help your institution during ‘normal’ times with improving healthcare by deploying AI and machine learning.
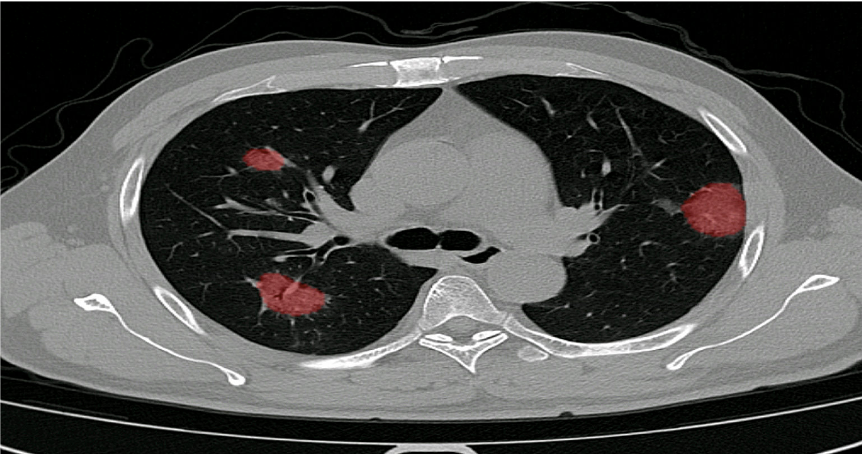
In December 2019, we started clinical validation of PulmoFast, the game-changing tool for computer-assisted detection for pulmonary nodules on CT-scans on the chest. Together with The Maasstad Hospital (Rotterdam, The Netherlands), we are assessing various KPI’s, which have so have been to exceeding expectations.
Furthermore, PulmoFast is currently undergoing regulatory approval in Europe (CE) and the US (FDA).
PulmoFast will be rolled out for further clinical validation in the coming months. After obtaining regulatory approval, PulmoFast will be made available at a competitive transaction-based pricing model, creating greater value whilst simultaneously reducing costs, creating a leap in value for doctors, hospitals and society.