The Maasstad Ziekenhuis (Rotterdam, The Netherlands) will use Artificial Intelligence (AI) to quantify the amount of involved tissue in patients that are possibly infected with the Coronavirus (COVID-19). This is done in close collaboration with Holland AI, a company active in the development and integration of AI-applications in healthcare.
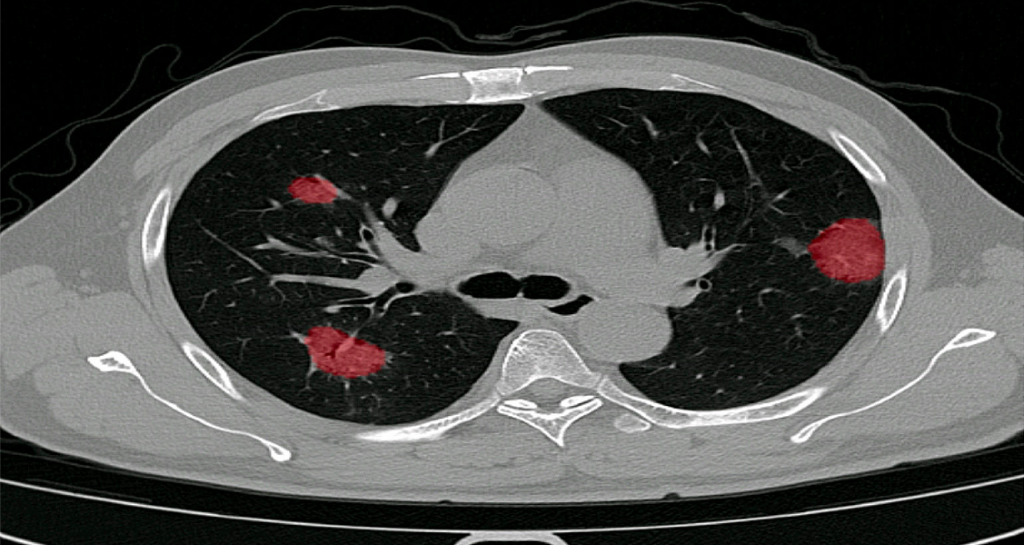
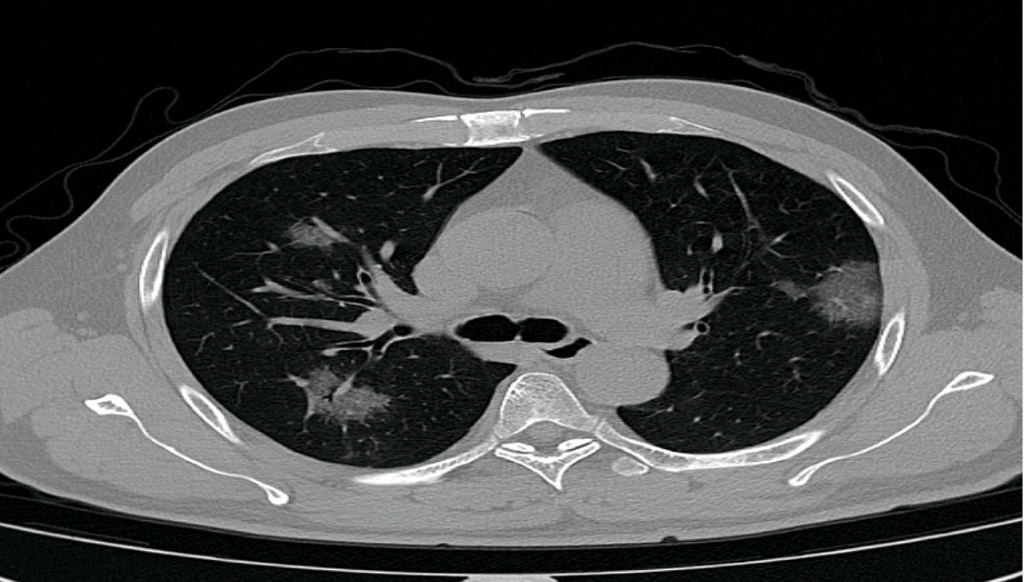
The application, PulmoFast-CTSS, analyzes chest CT-scans and gives an indicative score (CT severity score) of the amount of involved tissue. Previous studies have shown prognostic value of the CT severity score, and thus the score may help clinicians in their treatment choices. The Maasstad Hospital and Holland AI are now working on a clinical study to further evaluate the value of the CT severity score. Based on the outcomes of their analyses, further steps will be taken, for example distribution of knowledge and the application to other hospitals.
“Several companies are joining forces and are freely offering their AI software for research of and the fight against the coronavirus. Just as Holland AI, a start-up company of one of our residents Ayoub Charehbili and his business partner Damiaan Sprenger”, says André Niezen, a radiologist in the Maasstad Ziekenhuis. “The department of radiology and nuclear medicine of the Maasstad Ziekenhuis is proud to – in addition to regular diagnostics – contribute to the diagnosis and treatment of patients contaminated with the coronavirus (COVID-19).”
André Niezen, a radiologist in the Maasstad Ziekenhuis
AI in a supportive role
The radiologist is and stays the person responsible for the analysis of the CT-images, but can be assisted by PulmoFast-CTSS. To make this possible, the AI-software is fully integrated within the existing IT-infrastructure.
Multidisciplinary collaboration
The Maasstad Ziekenhuis will also investigate if it’s possible to integrate the CT-severity score in other predictionmodels (for example with lab test values). For this reason the hospital installed a multidisciplinary crisis workgroup on AI of multiple clinical and diagnostic specialisms, the clinical-chemical laboratory and the department of clinical informatics.
In December 2019, we started clinical validation of PulmoFast, the game-changing tool for computer-assisted detection for pulmonary nodules on CT-scans on the chest. Together with The Maasstad Hospital (Rotterdam, The Netherlands), we are assessing various KPI’s, which have so have been to exceeding expectations.
Furthermore, PulmoFast is currently undergoing regulatory approval in Europe (CE) and the US (FDA).
PulmoFast will be rolled out for further clinical validation in the coming months. After obtaining regulatory approval, PulmoFast will be made available at a competitive transaction-based pricing model, creating greater value whilst simultaneously reducing costs, creating a leap in value for doctors, hospitals and society.